Showing CypComp Card for CC02057: Ticlopidine (CypBoM Training Set)
| Record Information | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||
| Creation Date | 2019-07-14 16:36:34 UTC | ||||||||||||||||||
| Last Updated | 2019-10-01 21:41:21 UTC | ||||||||||||||||||
| CypComp ID | CC02057 | ||||||||||||||||||
| Compound Information | |||||||||||||||||||
| Name | Ticlopidine | ||||||||||||||||||
| Structure Image | 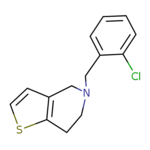 | ||||||||||||||||||
| InChIKey | PHWBOXQYWZNQIN-UHFFFAOYSA-N | ||||||||||||||||||
| PubChem ID | 5472 | ||||||||||||||||||
| CypCompound Information | |||||||||||||||||||
| Set | CypBoM Training Set | ||||||||||||||||||
| Bonds of Metabolism (BoMs) |
| ||||||||||||||||||
| References |
| ||||||||||||||||||
| Download File | CC02057.sdf | ||||||||||||||||||